Learning Outcomes
By the end of this lesson, students should be able to:
i. Define carboxylic acid derivatives and their general characteristics.
ii. Identify the different types of carboxylic acid derivatives: acyl halides, acid anhydrides, esters, and amides.
ii. Describe the methods for converting carboxylic acids into their respective derivatives.
iv. Explain the interconversion reactions between carboxylic acid derivatives.
v. Provide examples of carboxylic acid derivatives and their applications.
Introduction
Carboxylic acids, ubiquitous compounds in organic chemistry, are not limited to their acidic nature. They serve as versatile precursors for a wide range of derivatives, each with its unique properties and reactivity. This lesson delves into the fascinating world of carboxylic acid derivatives, exploring their synthesis, interconversions, and applications.
i. Carboxylic Acid Derivatives: A Diverse Family
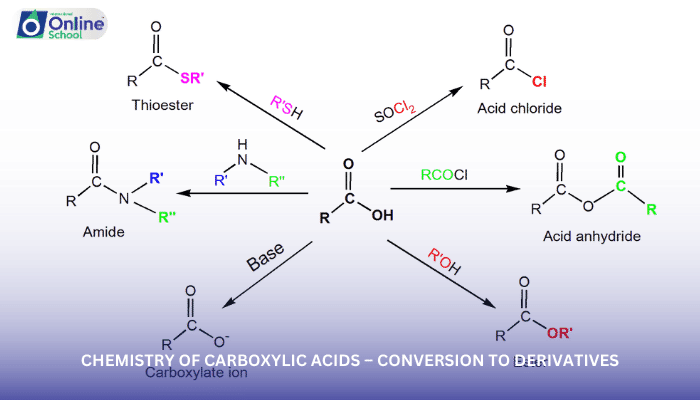
Carboxylic acid derivatives are a diverse group of compounds obtained by replacing the hydroxyl group (-OH) of the carboxylic acid with a different functional group. Each derivative exhibits unique properties and reactivity, expanding the chemical landscape of carboxylic acids.
ii. Acyl Halides: The Reactive Counterparts
Acyl halides, also known as acid chlorides, are characterized by the presence of a halogen atom (-Cl, -Br, or -I) bonded to the carbonyl carbon. They are highly reactive and readily undergo nucleophilic substitution reactions.
iii. Acid Anhydrides: The Double-Edged Sword
Acid anhydrides, containing two carbonyl groups linked by an oxygen atom, are formed by the dehydration of carboxylic acids. They act as versatile acylating agents, capable of transferring an acyl group to nucleophiles.
iv. Esters: The Sweet and Fragrant Enchantment
Esters, the most common carboxylic acid derivative, are formed by the condensation of a carboxylic acid with an alcohol. They are found abundantly in nature, contributing to the flavors and aromas of fruits, flowers, and other natural products.
v. Amides: The Nitrogen-Containing Champions
Amides, characterized by the carbonyl carbon bonded to a nitrogen atom, are derived from the reaction of carboxylic acids with ammonia or amines. They play crucial roles in biochemistry, forming the backbone of proteins and participating in various enzymatic reactions.
vi. Interconversion Reactions: A Dynamic Dance
Carboxylic acid derivatives can undergo interconversion reactions, transforming one derivative into another. These reactions often involve nucleophilic acyl substitution, highlighting the reactivity of the carbonyl carbon.
For instance, acyl halides can be converted to esters through reaction with alcohols, while acid anhydrides can react with ammonia to produce amides. These interconversions demonstrate the interconnectedness of carboxylic acid derivatives.
vii. Applications: A World of Possibilities
Carboxylic acid derivatives find diverse applications in various fields, including:
Esters: Used as flavorings, fragrances, solvents, and plasticizers
Amides: Found in proteins, pharmaceuticals, and nylon
Acyl Halides: Employed in organic synthesis and dye production
Acid Anhydrides: Used as curing agents for resins and as intermediates in polymer synthesis
Carboxylic acid derivatives, with their varied structures and properties, expand the versatility of carboxylic acids. Their synthesis, interconversion reactions, and wide range of applications showcase the rich chemistry of these compounds. Understanding the chemistry of carboxylic acid derivatives is essential for organic synthesis, biochemistry, and various industrial processes.